A probe of steric ligand substituent effects on the spin crossover of Fe(ii) complexes†
Abstract
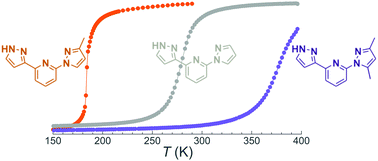
Identifying and quantifying the individual factors affecting the temperature and properties of the spin crossover in transition metal complexes is a challenging task, because many variables are involved. While the most decisive factor is the crystal field imparted by ligands around the active metal center, some less common actors are intramolecular steric repulsions or non-covalent interactions. A series of three Fe(II) complexes of 1,3bpp derivatives of (2-(pyrazol-1-yl)-6-(1H-pyrazol-3-yl)pyridine) have been prepared and characterized crystallographically to probe these effects: [Fe(1,3bpp)2](ClO4)2 (1), [Fe(met1,3bpp)2](ClO4)2 (2) and [Fe(dimet1,3bpp)2](ClO4)2 (3). The ligands exhibit none, one or two methyl substituents on the pyrazol-1-yl heterocycle. These groups exert a dramatic effect on the SCO temperature in the solid state, and, most significantly, in solution (with TSCO (3) > TSCO (1) > TSCO (2)). Extensive DFT calculations have unveiled the origin of these effects which lie in the intramolecular non-covalent or steric interactions rather than resulting from crystal field effects.


 Please wait while we load your content...
Please wait while we load your content...