A carbon based drug delivery system derived from a one-dimensional coordination polymer, doxorubicin loading and redox-responsive release†
Abstract
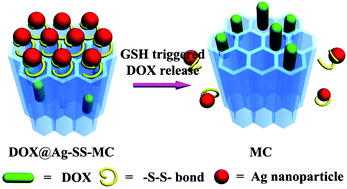
Since the controlled release of drug molecules under external stimuli is an essential strategy for effective tumor therapy, herein a redox-responsive drug delivery system (Ag-SS-MC) has been fabricated successfully through the modification of disulfide bonds on coordination polymer derived mesoporous carbon nanoparticles (MC) with nanoscale Ag as the “cap”. In this drug delivery system, the Ag “caps” are fastened on a disulfide linker tightly, which blocks the pores of MC and impedes unexpected leakage of loaded doxorubicin (DOX). This drug delivery system exhibits a striking DOX loading property, with loading ratio as high as 20.6%. Ag-SS-MC remains stable under normal physiological conditions, but the loaded DOX begins to be released in the presence of a reducing agent, glutathione (GSH), which is caused by reductive breaking of disulfide bonds. MTT assays show that, after incubation with the human cervical cancer cell line (HeLa) for 24 h, the cytotoxicity of blank Ag-SS-MC carriers can almost be ignored. In contrast, under the same conditions, the DOX loaded drug carrier system possesses high antitumor activity. Ag-SS-MC exhibits superior drug loading, high extracellular stability, redox-responsive intracellular drug release and excellent biocompatibility; all these characteristics make it a promising drug delivery system for tumor therapy.


 Please wait while we load your content...
Please wait while we load your content...