Investigation of redox switchable titanium and zirconium catalysts for the ring opening polymerization of cyclic esters and epoxides†
Abstract
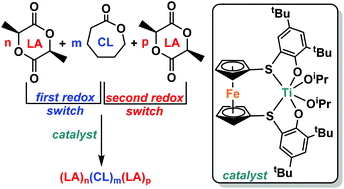
The synthesis and characterization of (thiolfan*)Zr(OtBu)2 (thiolfan* = 1,1′-di(2,4-di-tert-butyl-6-thiophenoxide)ferrocene) is reported, as well as its activity toward the ring-opening polymerizations of L-lactide and ε-caprolactone. With the titanium analogue, (thiolfan*)Ti(OiPr)2, diblock copolymers (AB and BA) and a triblock copolymer (ABA) were synthesized in a one-pot, redox-controlled process. Changing the metal center from titanium to zirconium has a profound influence on the reactivity profile of the corresponding reduced and oxidized catalysts in the switchable ring opening polymerization of cyclic esters and ethers.
- This article is part of the themed collections: Celebrating Excellence in Research: 100 Women of Chemistry, Celebrating Excellence in Research: Women at the Frontiers of Chemistry and Switchable Catalysis and Related Reactions


 Please wait while we load your content...
Please wait while we load your content...