Cadmium(ii) coordination polymers based on substituted malonic acid: synthesis, characterization and photoluminescence properties†
Abstract
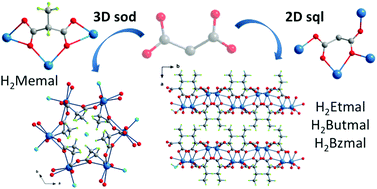
Four novel complexes of cadmium(II) with alkyl/aryl-substituted malonate ligands of formulae {[Cd(Memal)(H2O)]6·0.5H2O}n (1), [Cd(Etmal)(H2O)]n (2), [Cd(Butmal)(H2O)]n (3) and [Cd(Bzmal)(H2O)]n (4), (Memal = methylmalonate, Etmal = ethylmalonate, Butmal = butylmalonate and Bzmal = benzylmalonate) have been prepared and characterized by single crystal X-ray analysis. Their luminescence, UV-Vis absorption properties and thermal behaviour were also investigated. Complex 1 is a three-dimensional compound where each metal centre is connected to four other ones leading to a sodalite network with the point symbol {42·64}. Each cadmium(II) ion in 1 is seven-coordinate with a water molecule and six oxygen atoms from three Memal ligands building a somewhat distorted pentagonal bipyramidal surrounding. The Memal ligand in 1 adopts a μ3–κO:κ2O′:κ2O′′:κO′′′ coordination mode. 2–4 are isomorphous compounds with a two-dimensional structure where centrosymmetric double oxo(carboxylate-substituted malonate)-bridged dicadmium(II) units are linked to four other dinuclear entities through anti–anti and anti–syn carboxylate bridges to form a Shubnikov tetragonal layered [4462]-sql net. Each cadmium(II) ion in 2–4 is six-coordinate in a distorted octahedral environment with four carboxylate-oxygen atoms building the basal plane and a water molecule plus another carboxylate-oxygen atom in the axial positions. The substituted malonate ligands in 2–4 exhibit the μ4–κO:κ2O′:κ2O′′:κO′′′ coordination mode. The tests of the emission spectra of 1–4 show that the length of the side chain influences the photoluminescence properties of the complexes. Finally, the decays of the luminescence of 1–4 and the respective free substituted malonic acids reveal that the same value of the average life time is observed for 1 (H2Memal), whereas in the case of 2–4, they are greater than that of 1 and those of their corresponding acids. The longer decay rates in 2–4 are consistent with the lack of water molecules in the environment of the cadmium(II) ion in these complexes, indicating that the sheet-like structure of 2–4 provides a chemical surrounding with less non-radiative relaxation processes.


 Please wait while we load your content...
Please wait while we load your content...