Stoichiometry of lanthanide(iii) complexes with tripodal aminophosphonic ligands – a new solution to an old problem†
Abstract
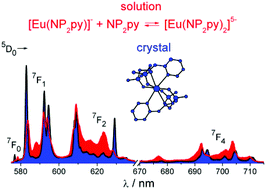
The Eu3+ and Gd3+ complexes with an N-(methylene-2-pyridine)-N,N-di(methylenephosphonate) ligand (H4NP2py), an analogue of nitrilotri(methylphosphonic) acid (H6NTP), were synthesized and structurally characterized by X-ray single crystal diffraction. The determined crystal structures ([C(NH2)3]5[Ln(NP2py)2]·12H2O) are the first example of a monomeric Ln3+ complex encapsulated by two tripodal aminophosphonic ligands. Each of the NP2py anions coordinates to Ln3+ through two oxygen atoms from each monodentate phosphonic group, amine nitrogen and pyridine nitrogen atoms, filling thus 8 coordination sites of Ln3+. The luminescence properties of [C(NH2)3]5[Eu(NP2py)2]·12H2O crystals were studied and compared with those of Eu–NP2py complexes in solution. Speciation analysis of Ln–NP2py complexes (Ln : NP2py = 1 : 2), performed by luminescence and potentiometric methods, showed that both [Ln(NP2py)]− and [Ln(NP2py)2]5− species may exist in solution. However, the formation of the latter one occurs in alkaline solutions at pH as high as 8. By implementing the Specific Ion Interaction Theory (SIT) it was possible to calculate the thermodynamic stability constants of the [Eu(NP2py)]− and [Eu(NP2py)2]5− complexes. The corresponding log β0Eul and 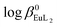 values are 16.3 ± 0.11 and 19.5 ± 0.15, respectively.
values are 16.3 ± 0.11 and 19.5 ± 0.15, respectively.

 Please wait while we load your content...
Please wait while we load your content...