A redox-switchable ring-closing metathesis catalyst†
Abstract
A Ru(II) complex ligated to a quinone-annulated N-heterocyclic carbene (NHC) was synthesized as a redox-active analogue of the Hoveyda–Grubbs II generation catalyst. The complex exhibited a single reversible reduction with a E1/2 of −0.63 V (vs. SCE), and was successfully reduced and then oxidized with high fidelity using chemical reagents. While the catalyst facilitated a range of ring-closing metathesis (RCM) reactions in its neutral state, its activity was inhibited upon the introduction of a suitable reducing reagent. A series of density functional theory calculations revealed that the differences in catalytic activity may be attributed to the stronger donating ability of the reduced NHC ligand which stabilized a ruthenacyclobutane intermediate and thus suppressed the rate-determining retro-[2 + 2] cycloaddition step of the underlying RCM mechanism.
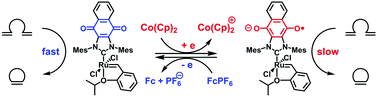
- This article is part of the themed collection: Switchable Catalysis and Related Reactions


 Please wait while we load your content...
Please wait while we load your content...