Solvent-controlled syntheses of mixed-alkali-metal borates exhibiting UV nonlinear optical properties†
Abstract
The mixed-alkali-metal borates NaK(B5O8)(OH)·H2O (1), NaK6[(B4O5)(OH)4]3(OH)·C2H5OH (2) and Na0.33K1.67(B4O5)(OH)4·3H2O (3) have been solvothermally synthesized using various polar organic solvents. Compounds 1 and 2 crystallize in the centrosymmetric space groups P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and R
and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, respectively. The structure of 1 features a 2D-layered framework constructed by [B5O11]7− primary building units in the ab plane, extending to a 3D framework linked by K+ and Na+ cations, while the structure of 2 can be described as isolated [B4O9]6− primary building units connected by H-bonding interactions and K–O and Na–O bonds, forming a 3D supramolecular framework. Compound 3 crystallizes in the acentric space group P
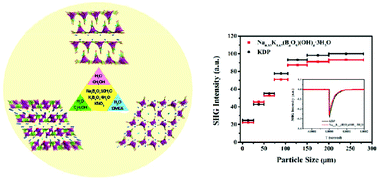
c, respectively. The structure of 1 features a 2D-layered framework constructed by [B5O11]7− primary building units in the ab plane, extending to a 3D framework linked by K+ and Na+ cations, while the structure of 2 can be described as isolated [B4O9]6− primary building units connected by H-bonding interactions and K–O and Na–O bonds, forming a 3D supramolecular framework. Compound 3 crystallizes in the acentric space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c, and its UV nonlinear optical properties have been investigated for the first time. Second-harmonic generation (SHG) measurements show that 3 is type-I phase-matching, with a moderate SHG response ca. 0.94 times that of KH2PO4. The cut-off edge of 3 is 242 nm, which suggests that 3 is a potential UV NLO material. Density functional theory calculations have been employed on 3 to rationalize its band structure and electron density as well as the density of states.
2c, and its UV nonlinear optical properties have been investigated for the first time. Second-harmonic generation (SHG) measurements show that 3 is type-I phase-matching, with a moderate SHG response ca. 0.94 times that of KH2PO4. The cut-off edge of 3 is 242 nm, which suggests that 3 is a potential UV NLO material. Density functional theory calculations have been employed on 3 to rationalize its band structure and electron density as well as the density of states.


 Please wait while we load your content...
Please wait while we load your content...