Blue and orange oxygen responsive emissions in the solid state based on copper(i) complexes bearing dodecafluorinated diphosphine and 1,10-phenanthroline derivative ligands†
Abstract
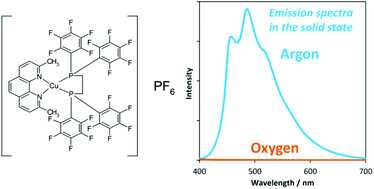
Reversible oxygen sensing abilities based on blue and orange photoluminescence in the solid state are achieved by using newly synthesized copper(I) complexes bearing diimine and dodecafluorinated diphosphine ligands. We found that the blue emission of [Cu(dmp)(dfppe)]PF6 (dmp = 2,9-dimethyl-1,10-phenanthroline, dfppe = 1,2-bis[bis(pentafluorophenyl)phosphino]ethane) in the solid state is very strong under argon, while it is nearly invisible under air. Single crystal X-ray structural analysis reveals that the complex has significant columnar void spaces. [Cu(dmp)(dfppe)]PF6 shows an extremely long lifetime of the emission in the solid state under an argon atmosphere (τ1 = 160 μs (88%), τ2 = 22 μs (12%)), which is one of the largest values for all copper(I) complexes bearing diimine ligands, and it is drastically decreased under air (τ1 = 2.4 μs (85%), τ2 = 0.5 μs (15%)). The employment of the dfppe ligand markedly increases the contribution of ligand centred transition, leading to the long-lived excited state. The orange oxygen responsive emission of [Cu(47dmp)(dfppe)]PF6 (47dmp = 4,7-dimethyl-1,10-phenanthroline) is also examined with the help of an investigation of the photophysics of five new compounds of the copper(I) complexes.


 Please wait while we load your content...
Please wait while we load your content...