Heteronuclear assembly of Ni–Cu dithiolato complexes: synthesis, structures, and reactivity studies†
Abstract
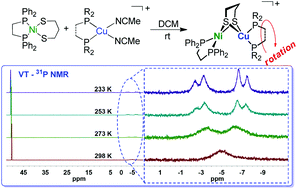
Heterometallic complexes exhibit the advantages of exceptional crystal structures and electronic performance over monometallic ones in mimicking the active site of metalloenzymes such as [NiFe] hydrogenase and acetyl-coenzyme A synthase/carbon monoxide dehydrogenase (ACS/CODH). Herein, we discovered a new mild route to synthesize [NiCu] binuclear complexes [(dppe)Ni(μ-pdt)Cu(dppe)](BF4) (dppe = 1,2-C2H4(PPh2)2, 1), [(dppe)Ni(μ-pdt)Cu(dppbz)]BF4 (dppbz = 1,2-C6H4(PPh2)2; 2), and [(dppe)Ni(μ-pdt)Cu(dcpe)]BF4 (dcpe = 1,2-C2H4(PCy2)2; 3) through Ni(pdt)(dppe) (pdt = (SC3H6S)2−) and [Cu(P–P)(NCMe)2]BF4 (P–P = diphosphine chelating ligand). The structures of these models in the [(dppe)Ni(pdt)Cu(P–P)]+ type are composed of Cu(I) in tetrahedral and Ni(II) in planar conformations. DFT calculations suggest that the HOMO corresponds to the delocalized π-orbital of the P–Cu–S system, while the LUMO is primarily composed of Ni, P, and S atoms with antibonding character.


 Please wait while we load your content...
Please wait while we load your content...