Unprecedented phthalocyanine–porphyrin-fused oligomers with induced chirality nature†
Abstract
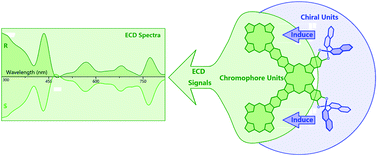
Optically active binaphthyl units have been introduced onto the periphery of the phthalocyanine chromophores in phthalocyanine–porphyrin-fused oligomers, resulting in the first (R)- and (S)-enantiomers of Pc–Por-fused dimer 1 and trimer 2 with induced chirality nature. This represents the largest chiral tetrapyrrole-based conjugated system, to the best of our knowledge. Electronic absorption, MCD, and CD spectroscopic results in combination with theoretical calculations disclose the effective transfer of the chiral information from the binaphthyl groups to both the central tetrapyrrole-fused chromophores, indicating the effective delocalization of the π-electron density over the fused systems due to the effective electronic communication between/among the tetrapyrrole chromophores.
- This article is part of the themed collection: HOT articles in Inorganic Chemistry Frontiers for 2016

 Please wait while we load your content...
Please wait while we load your content...