A comparative study of redox-active, ambipolar electrochromic triphenylamine-based polyimides prepared by electrochemical polymerization and conventional polycondensation methods†
Abstract
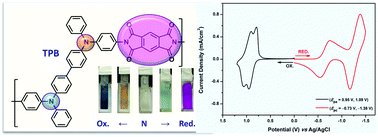
N,N′-Bis(4-diphenylaminophenyl)pyromellitimide (TPA-PMDI) was synthesized from the condensation reaction of 4-aminotriphenylamine with pyromellitic dianhydride (PMDA). The triphenylamine-diimide TPA-PMDI could be electrochemically polymerized into ambipolar, redox-active polyimide films on the electrode surface in an electrolyte solution via the coupling reactions between triphenylamine radical cations. The electro-generated polyimide films (coded as PI-E) exhibit reversible redox processes and stable color changes upon electro-oxidation, changing from a colorless neutral form to orange-yellowish and blue oxidized states. The film could also display pale green and pink cathodic coloring upon electro-reduction. Electrochromic devices using the electropolymerized film as an active layer were also fabricated as preliminary investigations for electrochromic applications. For a comparative study, a structurally similar polyimide (PI-C) was prepared by a conventional two-step condensation from N,N′-bis(4-aminophenyl)-N,N′-diphenyl-4,4′-biphenyldiamine with PMDA, and its spectroscopic, electrochemical and electrochromic properties were compared with those of the electro-synthesized PI-E.


 Please wait while we load your content...
Please wait while we load your content...