Synthesis of linear and star poly(ε-caprolactone) with controlled and high molecular weights via cyclic trimeric phosphazene base catalyzed ring-opening polymerization†
Abstract
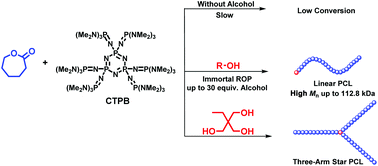
Poly(ε-caprolactones) (PCLs) with low catalyst loading and high molecular weights are highly desired considering their practical applications. In this work, cyclic trimeric phosphazene base (CTPB) has been developed and applied as an efficient organocatalyst in the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL). It is found that CTPB could directly catalyze the ROP of ε-CL but with a relatively low activity. In contrast, CTPB with alcohol as the initiator exhibits extremely high efficiency toward the ROP of ε-CL and rapidly converts hundreds even thousands equiv. monomers into PCLs with high conversions and controlled and high molecular weights. Both mono- and multi-hydroxy-alcohols are efficient as initiators, and thus PCLs with different topologies can be easily prepared. Moreover, with excess of alcohol, the title catalytic system represents a rare example of immortal polymerization by an organocatalyst for ε-CL.


 Please wait while we load your content...
Please wait while we load your content...