Visible-light responsive hydrogen-bonded supramolecular polymers based on ortho-tetrafluorinated azobenzene†
Abstract
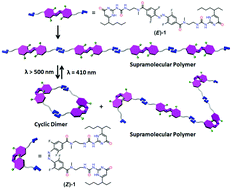
Novel visible-light responsive hydrogen-bonded supramolecular polymers have been successfully constructed by using a linear molecule, which consists of two ureido-pyrimidinone (UPy) moieties connected by a photochromic ortho-tetrafluorinated azobenzene (TFAB) linker. Benefiting from the visible-light responsive feature of the TFAB unit, reversible photoisomerization between the two isomers of the monomer can be achieved. The E-isomer prefers to form a linear supramolecular polymer through the intermolecular quadruple hydrogen bonding interactions between UPys, whereas the Z-isomer facilely forms a cyclic dimer. Therefore, the polymerization mechanisms and properties of such supramolecular polymers, e.g. reversible visible-light induced gel–sol transformation behaviour, can be regulated by the reversible visible-light triggered E/Z photo-isomerization of the TFAB chromophore.


 Please wait while we load your content...
Please wait while we load your content...