Recyclable and efficient polyurethane-Ir catalysts for direct borylation of aromatic compounds†
Abstract
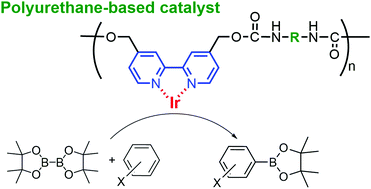
Four polyurethanes comprising 2,2′-bipyridyl moieties incorporated in the main chain were synthesized as a novel polymer ligand for the Ir(I)-catalyzed direct borylation of aromatic compounds. The polyurethanes were insoluble in common organic solvents and soluble in dimethylsulfoxide. The borylation reaction of benzene catalyzed by Ir(I) in the presence of the polymer prepared from 4,4′-bis(hydroxylmethyl)-2,2′-bipyridyl and 1,6-diisocyanatohexane led to the catalytic activities comparable to that in the presence of 2,2′-bipyridyl, while the polymers prepared by the reactions of 4,4′-bis(hydroxylmethyl)-2,2′-bipyridyl with 1,4-diisocyanatobenzene, 1,4-diisocyanato-3-methylbenzene, and bis(4-isocyanatophenyl)methane possesed lower catalytic activities. The high activity observed using the 1,6-diisocyanatohexane-based polyurethane catalyst could have a connection to the fact that this polymer has the highest tendency to form the inter-chain hydrogen bond. The borylation reaction systems with the polymers were biphasic, where the top and bottom layers contained the product and the polymer-based catalysts, respectively. Due to the phase separation, the product isolation and catalyst recycle were readily performed through a simple decantation. The catalyst prepared from 1,6-diisocyanatohexane-based polyurethane was able to be recycled at least five times without a significant decrease in activity. Further, the regio-selectivity in borylation of toluene, anisole, and trifluoromethybenzene was studied using the polyurethane ligands as well as the corresponding small-molecular ligands.


 Please wait while we load your content...
Please wait while we load your content...