Selective recognition of biologically important anions using a diblock polyfluorene–polythiophene conjugated polyelectrolyte†
Abstract
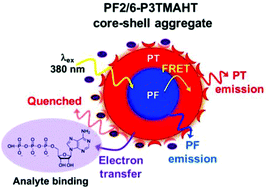
The all-conjugated diblock copolymer poly[9,9-bis(2-ethylhexyl)fluorene]-b-poly[3-(6-trimethylammoniumhexyl) thiophene] bromide (PF2/6-b-P3TMAHT) shows dual fluorescence from the polyfluorene (PF) and polythiophene (PT) blocks. Interaction with a range of nucleotide phosphates, at biologically relevant concentrations, was observed to bring about dramatic quenching of the PT emission, leaving the PF emission unaffected. The magnitude of this ratiometric response was found to depend on the number of phosphates attached to the nucleotide (guanosine triphophate (GTP) > guanosine diphphosphate (GDP) > guanosine monophosphate (GMP)), the type of nucleobase and the concentration. Modelling of the quenching behaviour and electrochemical studies suggest a mechanism involving energy transfer from the PF block to the PT block, followed by electron transfer from the PT to the nucleotides held in a Stern layer around the polymer core–shell aggregate that forms in water. Notably, the ability to discriminate between different numbers of phosphate groups is extremely relevant for energy metabolism processes. The enhanced insight into the quenching mechanism unravelled through this study provides the tools required to design targeted sensor platforms capable of discriminating different species through consideration of the size, charge and redox potential of the analyte.
- This article is part of the themed collection: Chemosensors and Molecular Logic


 Please wait while we load your content...
Please wait while we load your content...