Controlled poly(olefin)s via decarboxylation of poly(acrylic acid)†
Abstract
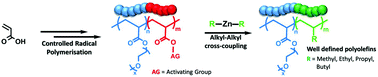
The preparation of polyolefins using controlled radical polymerisation (CRP) has long been a goal of polymer chemistry, but is hampered by the instability of olefin radicals. Herein we propose a simple strategy for the preparation of well-defined polyolefins such as polypropylene, by post-modification of poly(acrylic acid) with dialkylzinc reagents. The starting polymers can be readily synthesised by existing CRP techniques to almost any desired length and architecture. After activation of the carboxylic acid side chain and reaction with the dialkylzinc, a new C–C bond is formed between the alkyl group and the backbone carbon, and the carboxylic acid functionality is lost as CO2. We used this strategy to prepare well-defined polyolefins with methyl, ethyl, propyl and butyl side chains. As the dialkylzinc reagents are unreactive towards esters we were also able to use this approach to synthesise and self-assemble a range of PEGMEA-olefin block copolymers.


 Please wait while we load your content...
Please wait while we load your content...