Cationic disulfide-functionalized worm gels†
Abstract
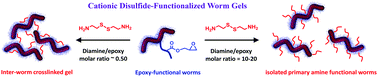
The recent development of polymerization-induced self-assembly (PISA) has facilitated the rational synthesis of a range of diblock copolymer worms, which hitherto could only be prepared via traditional post-polymerization processing in dilute solution. Herein we explore a new synthetic route to aqueous dispersions of cationic disulfide-functionalized worm gels. This is achieved via the PISA synthesis of poly[(glycerol monomethacrylate-stat-glycidyl methacrylate)]-block-poly(2-hydroxypropyl methacrylate) (P(GMA-stat-GlyMA)-PHPMA) block copolymer worms via reversible addition–fragmentation chain transfer (RAFT) aqueous dispersion polymerization of HPMA. A water-soluble reagent, cystamine, is then reacted with the pendent epoxy groups located within the P(GMA-stat-GlyMA) stabilizer chains to introduce disulfide functionality, while simultaneously conferring cationic character via formation of secondary amine groups. Moreover, systematic variation of the cystamine/epoxy molar ratio enables either chemically cross-linked worm gels or physical (linear) primary amine-functionalized disulfide-based worm gels to be obtained. These new worm gels were characterized using gel permeation chromatography, 1H NMR spectroscopy, transmission electron microscopy, dynamic light scattering, aqueous electrophoresis and rheology. In principle, such hydrogels may offer enhanced mucoadhesive properties.


 Please wait while we load your content...
Please wait while we load your content...