The “bottom-up” construction of chiral porous organic polymers for heterogeneous asymmetric organocatalysis: MacMillan catalyst built-in nanoporous organic frameworks†
Abstract
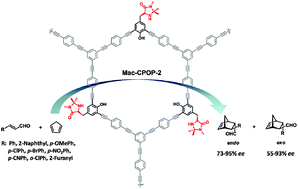
Although many porous organic polymers (POPs) embedded with chiral ligand–metal catalytic activities have been examined as heterogeneous asymmetric catalysts, examples of metal-free stereoselective POP catalysts are very scarce. Herein, we report a “bottom-up” strategy used to construct a MacMillan catalyst-embedded chiral porous organic polymer, Mac-CPOPs. The porosity of the Mac-CPOPs frameworks could be adjusted by varying the molecular length of the rigid structural monomers. Due to the high Brunauer–Emmett–Teller specific surface area and the built-in character of the covalently linked MacMillan catalyst, the Mac-CPOP-2 polymer can be applied as a highly efficient and recoverable heterogeneous organocatalyst in the asymmetric Diels–Alder reaction, which gives products in good yields and with good enantioselectivity. Moreover, the Mac-CPOP-2 polymer can be reused 6 times for the asymmetric Diels–Alder reaction without any significant loss of catalytic activity and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...