Polymerization of 1-chloro-2-benzaldehyde-acetylene using an NHC-Pd/AgOTf catalyst and post-polymerization modification†
Abstract
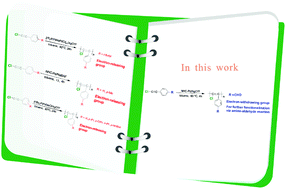
We report the synthesis of a poly(1-chloro-2-phenylacetylene) derivative with an electron-withdrawing substituent on the phenyl group. A combination of an N-heterocyclic carbene-based Pd catalyst (NHC-Pd) with silver trifluoromethyl sulfonate (AgOTf) formed an active catalytic system for the polymerization of 1-chloro-2-(para-substituted)-phenylacetylenes. Based on this active catalytic system, functional poly(di-substituted acetylene) (PDSA) bearing an aldehyde group (P3) was obtained in high yield. The exceptional P3 bearing a reactive aldehyde group on its side chain was used as a precursor to be subsequently modified with amines to generate a novel PDSA (P3-ppm) possessing Schiff-base functionalities in good yield.


 Please wait while we load your content...
Please wait while we load your content...