Alkyl-substituted spiropyrans: electronic effects, model compounds and synthesis of aliphatic main-chain copolymers†
Abstract
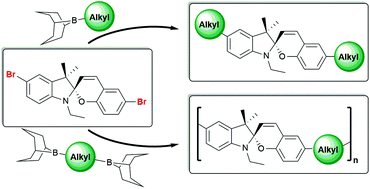
The isomerisation behaviour of spiropyrans (SP) depends considerably on their substituents. This work investigates the synthesis, isomerisation behaviour and polymer chemistry of alkylated spiropyrans. While several Kumada- and Suzuki-based protocols failed to alkylate dibromo-spiropyran, classical 9-BBN chemistry is shown to proceed with high yield suitable also for polycondensation. Alkyl substituents strongly influence the acid-induced SP-merocyanine (MC)-protonated MC (MCH+) equilibrium and render the MCH+ form most stable. In agreement with measured isomerisation rates, density functional theory calculations corroborate the enhanced stabilisation of ethyl-substituted MCH+ as compared to phenyl-substituted MCH+. Aliphatic main chain spiropyran copolymers are synthesised from in situ generated bis-9-BBN monomers and dibromo-spiropyrans. The empirical optimisation of stoichiometry yields a molecular weight Mw of ∼15 kg mol−1 after purification. This approach to alkylated spiropyrans opens new opportunities to fine-tune the isomerisation behaviour of small molecules and polymeric SP derivatives for potential use as sensors and smart materials.


 Please wait while we load your content...
Please wait while we load your content...