Conjugated phenothiazine oxime esters as free radical photoinitiators†
Abstract
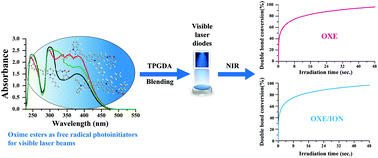
Three novel phenothiazine-substituted oxime esters (Ph-PTZ-OXE, TPA-PTZ-OXE and CZ-PTZ-OXE) applicable to visible laser diodes (405 nm and 455 nm) were successfully designed and synthesised. The introduction of phenothiazine groups in oxime esters led to a significant redshift in UV-vis absorption, thereby making the oxime esters suitable for promoting photopolymerization under visible light irradiation. Upon exposure to the irradiation of a laser diode at 405 nm and 455 nm, the three oxime esters showed high polymerization efficiency in the free radical polymerization of tripropylene glycol diacrylate (TPGDA). The oxime ester system was paired with iodonium salt (ION) and exhibited high efficiency in promoting free radical polymerization. The photochemical mechanisms were established from the fluorescence spectrum, cyclic voltammograms (CV) and electron spin resonance (ESR) data. The iminyl radical is characterized by two quartets centered at 323.1 mT and 324.4 mT. The benzoyloxyl radical gives rise to two broad peaks at 320.9 mT and 336.7 mT, with a high g value characteristic of oxygen-centered radicals.


 Please wait while we load your content...
Please wait while we load your content...