Phosphazene/triisobutylaluminum-promoted anionic ring-opening polymerization of 1,2-epoxybutane initiated by secondary carbamates†
Abstract
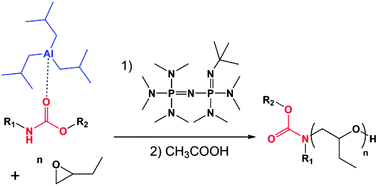
Attempts to use a carbamate-phosphazene base as the initiating system for the polymerization of 1,2-epoxybutane was unsuccessful. As a matter of fact, carbamate deprotonation by phosphazene bases led to their fast decomposition generating alkoxide anions which initiate the polymerization rather than carbamate anions. Conversely, in the presence of triisobutylaluminum – a Lewis acid – the in situ generation of an anionic initiator X− obtained by the deprotonation of the tBuP4 phosphazene base was tested as a possible way to initiate the polymerization of 1,2-epoxybutane. Particular attention was given to the detection of eventual transfer or side-reactions according to the carbamate : triisobutylaluminum : phosphazene base ratio, to the solvent dielectric constant and to the number of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– units in the phosphazene base. The reaction was performed with a stoichiometric ratio (1 : 1 : 1) of carbamate : triisobutylaluminum : tBuP2, which gave the best results. Under these conditions, the initiation of the polymerization by the carbamate anion was quantitative; no transfer reactions have been observed and the polymerization proceeded in a controlled manner to afford amide end-capped poly(butylene oxide) with a narrow molar mass distribution and expected molar masses.
N– units in the phosphazene base. The reaction was performed with a stoichiometric ratio (1 : 1 : 1) of carbamate : triisobutylaluminum : tBuP2, which gave the best results. Under these conditions, the initiation of the polymerization by the carbamate anion was quantitative; no transfer reactions have been observed and the polymerization proceeded in a controlled manner to afford amide end-capped poly(butylene oxide) with a narrow molar mass distribution and expected molar masses.


 Please wait while we load your content...
Please wait while we load your content...