Tunable amphiphilic graft copolymers bearing fatty chains and polyoxazoline: synthesis and self-assembly behavior in solution†
Abstract
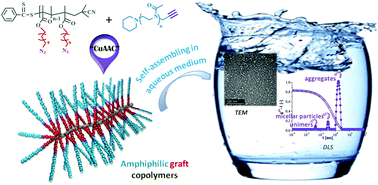
Amphiphilic graft copolymers composed of a methacrylate-based hydrophobic backbone with C11 pending chains end-capped by hydrophilic polyoxazoline (POx) blocks were successfully synthesized using the grafting-onto method by associating reverse addition fragmentation chain transfer (RAFT) polymerization with azide–alkyne Huisgen's cycloaddition (CuAAC). Pure and well-characterized copolymers with different molecular weights and without traces of starting polymers were obtained. The hydrophilic character of the copolymers was controlled by copolymerizing the azido-terminated monomer 11-azido-undecanoyl methacrylate (AzUMA) with lauryl methacrylate (LM), the corresponding (co)polymers being then coupled with α-alkyne-POx. Using dynamic light scattering (DLS) and transmission electronic microscopy (TEM) techniques, their self-assembling behavior was studied by direct dissolution in water without further filtration. Depending on the systems, two or three particle size distributions co-exist in solution. However, the smallest objects, with DH-values around 6, 42 and 255 nm, were obtained with the most hydrophilic copolymers (∼90% of hydrophilic group per molecule). Moreover, the influence of the starting copolymer architecture on its self-organization behavior was emphasized by comparing the self-assembly of the graft copolymers with that of an amphiphilic macromonomer, in situ polymerized, and whose structure mimics that of the repeating unit of the graft copolymers.


 Please wait while we load your content...
Please wait while we load your content...