All-conjugated P3HT donor PCDTBT acceptor graft copolymers synthesised via a grafting through approach†
Abstract
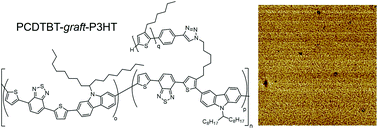
A generally applicable synthetic method for all-conjugated graft copolymers is presented. By using an PCDTBT acceptor backbone and poly(3-hexylthiophene) (P3HT) grafts, all conjugated graft copolymers are synthesized using a grafting-through strategy which permits independent control over the graft length, graft density and backbone length. A TBT-P3HT macromonomer is synthesized using Suzuki coupling of H-P3HT-Br to yield mono-ethynyl functionalized P3HT quantitatively. Mono-functionalized ethynyl-P3HT is subsequently converted into a TBTBr2-graft-P3HT macromonomer using click chemistry, which allows the attachment of the P3HT chains to the polymer backbone though flexible, non-conjugated alkyl chains. PCDTBT is made by a Suzuki polycondensation in the presence of TBTBr2-graft-P3HT (“grafting through”) to give well-defined graft copolymers whose grafting density is determined by the ratio of TBTBr2/TBTBr2-g-P3HT. High molecular weight products of the type PCDTBT-graft-P3HT that are free of any P3HT homopolymer are obtained, which exhibit superimposed absorption of the individual components and quenching of P3HT photoluminescence. P3HT in PCDTBT-graft-P3HT shows a similar degree of crystallinity to that of the macromonomer TBTBr2-graft-P3HT, indicating that most of the P3HT chains in PCDTBT-graft-P3HT are able to crystallize. Thermally annealed thin films exhibit a phase separated structure on the 10–20 nm length scale suitable for exciton diffusion and separation.


 Please wait while we load your content...
Please wait while we load your content...