Synthesis of photoresponsive main-chain oligomers with azobenzene moieties via ADMET oligomerization and their micellization properties†
Abstract
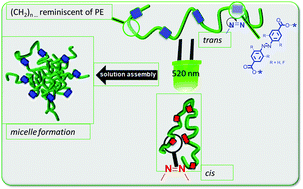
We report the synthesis of azobenzene functionalized linear unsaturated/saturated polyolefin-oligomers. Using mild reaction conditions and different ruthenium-based catalysts, the azobenzene moieties are precisely placed in olefin chains via acyclic diene metathesis (ADMET) reaction (Mn,SEC = 3.0 kDa–8.0 kDa). Subsequent hydrogenation reactions with p-toluenesulfonhydrazide yield the fully saturated azobenzene oligomers, which are thoroughly characterized by 1H-NMR, FTIR and liquid chromatography under critical conditions (LCCC). The obtained saturated and unsaturated azobenzene oligomers are proven to form micelles in solution, where their sizes and morphologies are characterized by DLS and TEM. As a result of photo-isomerisation from the trans to cis state, the aggregates reduce in size, which is accompanied by a visible change of the solution from turbid to clear.


 Please wait while we load your content...
Please wait while we load your content...