Solvated-electron initiated rapid polymerization at ambient-temperature: a case of monomer solubility†
Abstract
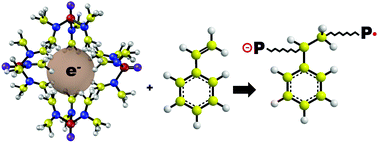
Solvated electrons are highly reactive species that offer a chance to develop odd-numbered electron chemistry in the form of a radical (1 electron) or a radical-anion (3 electrons). Although discovered more than half a century ago, their utility is encumbered by misunderstandings in monomer selectivity and inconsistencies in the polymerization mechanism. In this article, disagreements in the literature are re-examined, and through felicitous choice of reaction conditions (room temperature, partial monomer solubility, separation of reaction from undissolved metal), new insights were drawn from the polymerization of styrene and acrylate monomers. We observe that rapid polymerization gives polymers with significantly high molecular weights (Mw > 104 g mol−1, PDI < 2) in less than a minute. We note that negative inductive effects on an olefinic monomer are essential for polymerization, with olefins bearing unsubstituted hydrocarbons (positive inductive effects) failing to polymerize at all. We also demonstrate co-polymerization of styrene and methyl methacrylate, albeit with a significant bias towards the latter. We infer that the first species formed by electron transfer is a radical anion; however, the survival of the free radicals depends on the rate of de-solvation. Dimerization of the radical anions is promoted by a high local concentration of the initiated monomer. This process is highly dependent on enthalpy–entropy relations during the reaction–desolvation process, which we expound on using solubility and Hammett parameters.


 Please wait while we load your content...
Please wait while we load your content...