Photoinduced controlled radical polymerization of methacrylates with benzaldehyde derivatives as organic catalysts†
Abstract
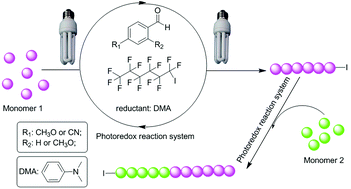
A photo-induced controlled radical polymerization of methacrylates with perfluoro-1-iodohexane as an initiator and benzaldehyde derivatives, including p-anisaldehyde, p-cyanobenzaldehyde and 2,4-dimethoxy benzaldehyde, as organic photocatalysts is demonstrated by using 23 W compact fluorescent lamps as the light source in the presence of a potential reductant N,N-dimethylaniline. Linear evolution of the molecular weight with monomer conversion is observed under the optimized conditions. Additionally, successful chain extension reactions are obtained by a one-pot process and with the as-prepared polymers as macroinitiators. Specifically, the one-pot synthesis of PPEGMA (conversion = 85%) with a high molecular weight and relatively narrow molecular weight distribution (Mn,GPC = 44 000 g mol−1, PDI = 1.54) is achieved by using PPEGMA-I (Mn,GPC = 10 200 g mol−1, PDI = 1.33) as a macroinitiator and p-anisaldehyde as an organocatalyst. Although the current technology exhibits a somewhat low controllability for preparing block copolymers, compared with the common ATRP and RAFT polymerization process, it offers a promising alternative as a metal-free organo-catalyzed photo-induced variant of ATRP.


 Please wait while we load your content...
Please wait while we load your content...