Diels–Alder cycloaddition and RAFT chain end functionality: an elegant route to fullerene end-capped polymers with control over molecular mass and architecture†
Abstract
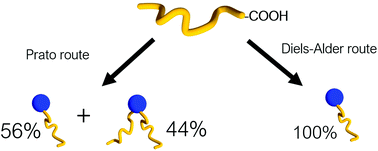
Fullerene C60 functionalised polymers (FFPs) have found numerous applications from photovoltaic devices to materials for photodynamic therapy. Polymer end-capping is one way to fabricate FFPs since it provides enhanced control over the macromolecular architecture and composition. This paper reports, for the first time, a facile, metal catalyst-free approach to FFPs where polymers, generated by reversible-addition fragmentation chain transfer (RAFT) polymerisation, were coupled to a fullerene derivative through chain-end functionality, provided by the chain transfer agent without further modification. Two routes to a fullerene derivative were compared – based on the Prato reaction and Diels–Alder cycloaddition. The Diels–Alder route exclusively yielded the mono-addition product, whereas the Prato route resulted in a mixture of mono- and diadducts which required further separation. This elegant combination of well-defined RAFT polymerisation and precise Diels–Alder addition allowed one to obtain fullerene end-capped polymers within a wide range of molecular masses (from 5000 to 50 000 g mol−1).


 Please wait while we load your content...
Please wait while we load your content...