Aziridine-functionalized polydimethylsiloxanes for tailorable polymeric scaffolds: aziridine as a clickable moiety for structural modification of materials†
Abstract
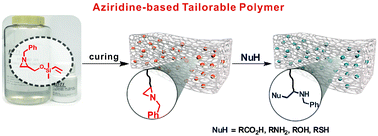
This paper shows that tailorable polymeric scaffolds on a molecular scale could be achieved through the ring opening reaction of a three-membered N-heterocyclic compound, aziridine. Aziridine is incorporated into an elastomeric polymer backbone (here, PDMS) through Pt(0)-catalyzed hydrosilylation, retaining attractive features such as optical transparency and elastic properties compared to conventional PDMS. The resulting aziridine-containing PDMS is chemoselectively and regio-specifically post-modified through an orthogonal ring opening reaction of the aziridine and takes advantage of the wide substrate scope of aziridine chemistry.


 Please wait while we load your content...
Please wait while we load your content...