A broad scope of aliphatic polyesters prepared by elimination of small molecules from sustainable 1,3-dioxolan-4-ones†
Abstract
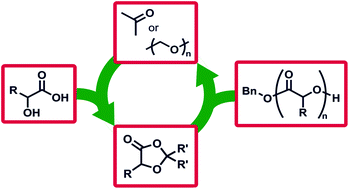
Biodegradable aliphatic polyesters built from sustainable feedstocks are one of the most promising solutions to address the pollution and oil-dependence challenges of modern plastics, but remain limited in monomer scope and thus the accessible polymer properties. We report a family of monomers that are built from renewable resources and use the elimination of small molecules to access aliphatic polyesters, circumventing challenging monomer syntheses to make these functionalised polymers. The driving force for ring opening polymerisation is the elimination of formaldehyde or acetone from easy-to-synthesise 1,3-dioxolan-4-ones to produce an array of structurally divergent polyesters. The polymers are prepared with high retention of stereochemistry, meaning isotactic polymers are easily prepared from natural enantiopure feedstocks. Reaction kinetics, structure/property relationships, copolymers of traditional cyclic esters, and direct recycling of waste paraformaldehyde showcase the scope of this new reaction in polymer chemistry.


 Please wait while we load your content...
Please wait while we load your content...