Synthesis of polyglycocarbonates through polycondensation of glucopyranosides with CO2†
Abstract
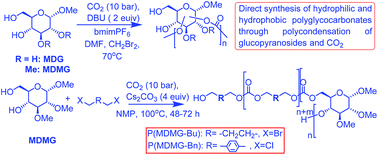
Starting from α-methyl D-glucopyranoside (MDG), three strategies of synthesis of polyglycocarbonates through direct polycondensation with CO2 were tried. Using unprotected MDG for reaction with CO2, water soluble oligoglycocarbonates could be obtained; α-methyl-2,3-di-O-methyl D-glucopyranoside (MDMG) which had its hydroxyls in the C2 and C3 positions protected was also subjected to polycondensation with CO2, affording polyglycocarbonates of limited molar mass due to an equilibrium that prevented the progress of the condensation reaction as in the previous case. Lastly, the polycondensation of MDMG with CO2 and aliphatic or aromatic dihalides was carried out in the presence of Cs2CO3; this resulted in the formation of polyglycocarbonates of rather high molar mass containing either aliphatic or aromatic linkers. The structures of the synthesized monomers and polyglycocarbonates were thoroughly characterized. The thermal properties of the obtained polyglycocarbonates were further investigated by TGA and DSC.


 Please wait while we load your content...
Please wait while we load your content...