Polymers from sugars and CO2: ring-opening polymerisation and copolymerisation of cyclic carbonates derived from 2-deoxy-d-ribose†
Abstract
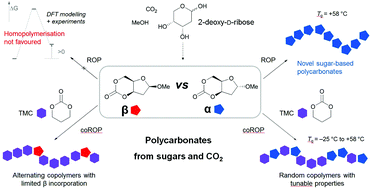
Bio-based aliphatic polycarbonates (APCs) are attractive synthetic materials for biomedical applications because of their biodegradabilty and biocompatability properties. A high yielding 3-step process that utilises CO2 as a C1 synthon is presented for converting raw sugar, 2-deoxy-D-ribose into a novel 6-membered cyclic carbonate for ring-opening polymerisation (ROP) into carbohydrate-based APCs. The α- and β-anomers of the monomer could be isolated and revealed very different polymerisability, as rationalised by DFT calculations. Whereas the β-anomer could not be polymerised under the conditions tested, organocatalytic homopolymerisation of the α-anomer, in solution at room temperature (rt) or under melt conditions, yielded highly insoluble polycarbonates, composed of both cyclic and linear topologies, and exhibiting a glass transition temperature (Tg) of ∼58 °C. Random copolymers with controllable incorporation of this new sugar monomer were prepared with trimethylene carbonate (TMC) at rt in the bulk or in solution with Mn up to 64 000 g mol−1. With increasing sugar content, the Tg values of the copolymers increased and their thermal degradability was enhanced, giving access to a new class of APCs with tailored properties.


 Please wait while we load your content...
Please wait while we load your content...