Preparation of sulfonic acid functional proton conducting phosphazenes by covalent protection†
Abstract
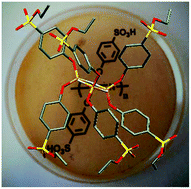
An easy and efficient method has been developed to prepare sulfonic acid functional cyclic and polymeric phosphazenes. Hexa(ethyl 4-oxybenzenesulfonate)cyclotriphosphazene and its sulfonic acid derivative, hexa(4-oxybenzenesulfonic acid)cyclotriphosphazene were successfully synthesized. Fully sulfonated polybis(4-oxybenzenesulfonic acid)phosphazene and three different copolymers composed of 4-methylphenol and 4-hydroxybenzenesulfonic acid have been prepared, in which the degree of sulfonation for whole polymers ranged from 25% to 100%. 1H, 31P NMR and FT-IR techniques were used to characterise the composition of the materials obtained by this new synthetic route. The structure of the hexa(ethyl 4-oxybenzenesulfonate)cyclotriphosphazene was also determined by single crystal X-ray diffraction. The thermal properties of the materials have been investigated by DSC and TGA, and the ion exchange properties were measured using volumetric titrations. The mechanical properties of the cast membranes were measured by using a universal tensile apparatus and compared with Nafion and post sulfonated aryloxyphosphazenes. Impedance spectroscopy was used to investigate the proton conductivity properties of the phenoxysulfonic acid substituted cyclic and polymeric phosphazenes at various temperatures. It was found that the proton conductivity of fully sulfonic acid functional polyaryloxyphosphazene is higher than that of Nafion under anhydrous conditions. Due to the advantages of this new synthetic route over the previous methods, it will be possible to prepare novel sulfonated phosphazene derivatives for different applications.


 Please wait while we load your content...
Please wait while we load your content...