“Clickable PEG” via anionic copolymerization of ethylene oxide and glycidyl propargyl ether†
Abstract
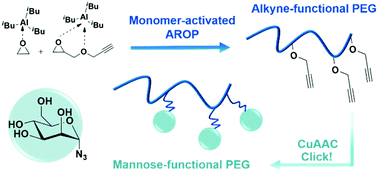
A straight forward synthesis of poly(ethylene glycol) (PEG) with multiple alkyne groups distributed along the polymer chain is introduced. Direct access to clickable PEG is achieved by the monomer-activated anionic ring-opening copolymerization (AROP) of ethylene oxide (EO) with glycidyl propargyl ether (GPgE). Notably for successful polymerization no protection of the alkyne unit is required owing to the mild reaction conditions. Defined PEG-co-PGPgE and PGPgE (co)polymers with PDIs of 1.18–1.60 and molecular weights of Mn = 3000–9500 g mol−1 were prepared. In situ1H NMR kinetic studies revealed remarkably disparate reactivity ratios of rEO = 14.8 and rGPgE = 0.076, representing a pronounced compositional drift with EO rich segments close to the initiator and GPgE units near the chain terminus, i.e. copolymers with a steep monomer gradient. Copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) with mannopyranosyl azide leads to PEG-based glycopolymers and highlights the potential of alkyne-functional PEGs as a universal scaffold for a range of biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...