Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization
Abstract
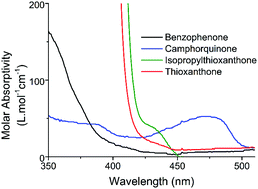
A novel methodology for photoinduced metal-free Atom Transfer Radical Polymerization (ATRP) by using conventional Type II photoinitiators such as benzophenone, thioxanthone, isopropyl thioxanthone and camphorquinone as sensitizers is presented. These sensitizers efficiently activate the ATRP of vinyl monomers when used in conjunction with co-initiators and alkyl halides under light irradiation. DFT calculations were performed to reveal mechanistic aspects and showed that the initiation of the controlled ATRP reaction pathway from the triplet state is energetically favoured for all sensitizers. In the case of benzophenone, however, the hydrogen abstraction pathway, which leads to free radical polymerization, is a possible side reaction. The strategy applied was proved to yield polymers with narrow molecular distribution. The chain-end fidelity of the polymer obtained was approved by chain extension and block copolymerization experiments, whereas the irradiation dependency of polymerization was confirmed by light on/off experiments.


 Please wait while we load your content...
Please wait while we load your content...