Poly(β-thioesters) containing monodisperse oxamide hard segments using a chemoselective thiol-Michael addition reaction†
Abstract
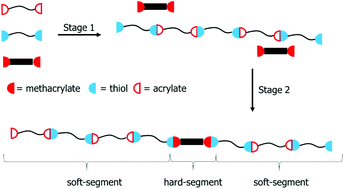
The inherent chemoselectivity of the thiol-Michael addition reaction enabled the synthesis of segmented copolymers using a one-pot, one-step procedure. The enhanced reactivity of a thiol towards an acrylate over a methacrylate generated thiol-terminated soft segment oligomers in the presence of bis-oxamide dimethacrylate hard segment monomers, furnishing segmented copolymers containing monodisperse hard segments after in situ chain extension. Thermomechanical characterization revealed extended plateau regions for all compositions with segmented and unsegmented copolymer analogs displaying similar plateau moduli, glass transition temperatures, and flow temperatures. Preliminary morphological analysis indicated a degree of undesired phase-mixing as a result of hydrogen-bond acceptors in the soft segment. The effectiveness of the bis-oxamide hydrogen-bonding motif allowed for sufficient phase separation to provide extended plateau regions for all samples as observed with dynamic mechanical analysis.


 Please wait while we load your content...
Please wait while we load your content...