Dual pH and ultrasound responsive nanoparticles with pH triggered surface charge-conversional properties†
Abstract
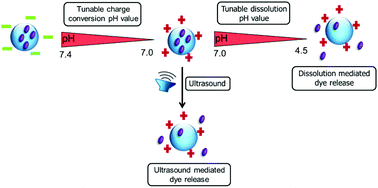
A series of dual pH- and ultrasound responsive statistical copolymers were synthesized via the reversible addition–fragmentation chain transfer (RAFT) polymerization of 3,4-dihydro-2H-pyran (DHP) protected HEMA 2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl methacrylate (THP-HEMA) and 2-(dimethylamino)ethyl methacrylate (DMAEMA). The RAFT-controlled nature of the (co)polymerizations was verified by detailed kinetic studies. The chemical structure and the co-monomer composition of the copolymers were confirmed by 1H NMR spectroscopy. The number-average molar mass values (Mn) and dispersities (ĐM = Mw/Mn) of the copolymers were estimated by size exclusion chromatography (SEC). The thermal properties of the (co)polymers were analyzed by means of thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Additionally, the DMAEMA moieties of the copolymers were quaternized with an excess of methyl iodide. The synthesized polymers self-assemble into nanoparticles in aqueous media via the nanoprecipitation method and were characterized by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Zeta potential measurements revealed that all DMAEMA containing nanoparticles undergo a surface charge conversion from positive to negative at slightly acidic pH values. However, quaternized DMAEMA nanoparticles possess pH independent positive surface charges. At acidic pH values, the nanoparticles disassemble and dissolve in water due to the protonation of the DMAEMA moieties and/or due to the acidic hydrolysis of the THP-HEMA groups. It was found that the surface charge and the stability of the nanoparticles were greatly affected by the DMAEMA content of the polymers, meaning that the isoelectric point (IEP), at which the charge is reversed and the pH value at which the disassembly occurs, increased with the higher DMAEMA content in the copolymer. Moreover, it was proven that the ionization of the carboxyl RAFT end-group of the polymers enhanced the anionic character and the stability of the nanoparticles at neutral pH values. DLS and scanning electron microscopy (SEM) measurements revealed that these nanoparticles can be further disrupted by ultrasound exposure. Nile Red was encapsulated into nanoparticles as a model hydrophobic drug. The release profile of the Nile Red was significantly accelerated in acidic media or under ultrasound exposure. The cytotoxicity assay results showed that negatively charged nanoparticles are non-toxic and biocompatible, whereas positively charged nanoparticles are extremely toxic to L929 cells.


 Please wait while we load your content...
Please wait while we load your content...