Search for a photoinduced (site-selective) cleavage of the Ar–Cl bond in dichloroanisoles†
Abstract
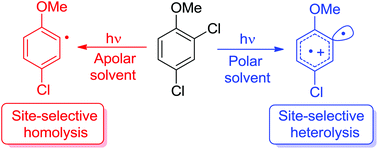
The site-selective cleavage of an Ar–X bond in polyhalogenated aromatics is an important tool in synthetic planning especially when more than one identical halogen atom is present. An alternative to the usual metal-catalyzed cleavage is represented by photochemistry although only a few examples have been reported. We then investigated the feasibility of the site-selective photodechlorination of some dichloroanisoles through a combined experimental and computational study. In the case of 2,4-dichloroanisole, selective detachment of the chlorine atom at the ortho position with respect to the OMe group was observed upon photohomolysis (in cyclohexane) or photoheterolysis (in MeOH) of the Ar–Cl bond. In the latter case, 5-chloro-2-methoxy-1,1′-biphenyl was exclusively formed upon reaction of the resulting phenyl cation with benzene. The substitution of an OH group for a OMe group was detrimental since a lower photoreactivity resulted with no improvement in the selectivity.


 Please wait while we load your content...
Please wait while we load your content...