3-Aminoperylene and ascorbate in aqueous SDS, one green laser flash … and action! Sustainably detoxifying a recalcitrant chloro-organic
Abstract
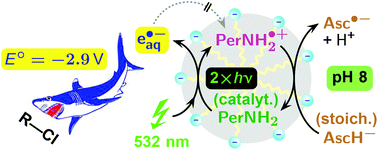
The hydrated electron represents a “super-reductant” in water, providing 2.9 eV of reductive power, which suffices to decompose nonactivated aliphatic halides. We show that 3-amino-perylene in SDS micelles, when combined with the bioavailable ascorbate as an extramicellar sacrificial donor, sustainably produces hydrated electrons through photoredox catalysis with green light, from a metal-free system, and at near-physiological pH. Photoionization of the amine with a 532 nm laser yields an extremely long-lived radical cation as the by-product, and a subsequent reaction of the latter with the sacrificial donor across the micelle/water interface regenerates the catalyst. The regeneration step involves parallel reactions between differently protonated forms, causing a bell-shaped pH dependence in basic medium. We have separated these processes kinetically. Employing this catalytic cycle for the laboratory-scale decomposition of chloroacetate, an accepted model compound for toxic and persistent halo-organic waste, gave turnover numbers of about 170. Even though both the substrate and the sacrificial donor compete for the hydrated electron, their consumption ratio is practically independent of the initial concentration ratio because the formal radical anion of the ascorbate undergoes secondary scavenging by the chloroacetate. In the course of the reaction, the initial hydrophobic catalyst is converted into a secondary species that is hydrophilic and still exhibits catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...