The effect of the functional ionic group of the viologen derivative on visible-light driven CO2 reduction to formic acid with the system consisting of water-soluble zinc porphyrin and formate dehydrogenase†
Abstract
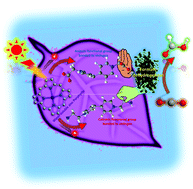
The effect of the functional ionic group of 4,4′-bipyridinium salt derivatives (4,4′-BPs) as the electron carrier on the visible-light driven conversion of CO2 to formic acid with the system consisting of water-soluble zinc tetraphenylporphyrin tetrasulfonate (ZnTPPS) and formate dehydrogenase (FDH) in the presence of triethanolamine (TEOA) as an electron donor was investigated. 1,1′-Diaminoethyl- (DAV), 1-aminoethyl-1′-methyl- (AMV), 1-carboxymethyl-1′-methyl- (CMV) and 1,1′-dicarboxymethyl-4,4′-bipyridinium salt (DCV) were prepared as the 4,4′-BPs with the functional ionic group. Irradiation of a CO2 saturated buffer solution containing TEOA, ZnTPPS, 4,4′-BP and FDH with visible light irradiation resulted in the production of formic acid. By using 4,4′-BPs with the cationic aminoethyl-group, DAV or AMV as an electron carrier, the effective visible-light driven formic acid production based on the CO2 reduction was observed compared to the 4,4′-BPs with the anionic carboxymethyl-group, CMV or DCV. The formic acid production rate with DAV was approximately 3.2 times higher than that of the system with DCV.


 Please wait while we load your content...
Please wait while we load your content...