Blue-shifted aggregation-induced emission of siloles by simple structural modification and their application as nitro explosive chemosensors†
Abstract
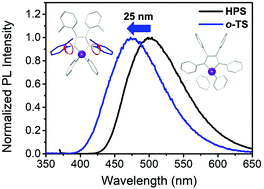
To induce blue-shifted emission of siloles, two tolyl-substituted derivatives – 1,1-diphenyl-2,3,4,5-tetra(m-tolyl)-1H-silole (m-TS) and 1,1-diphenyl-2,3,4,5-tetra(o-tolyl)-1H-silole (o-TS) – were prepared, and their photophysical properties were compared with those of a reference compound, hexaphenylsilole (HPS). By substituting methyl groups at ortho positions of peripheral tetraphenyl rings on the silacyclopentadiene ring, intramolecular rotations could be successfully controlled and the photophysical properties were varied, while substituting methyl groups at meta positions showed similar photophysical properties compared with the case of HPS. That is, simple structural modification at the ortho position significantly affects the geometry and the photophysical properties of silole, which leads to blue-shifted emission. Finally, two tolyl-substituted siloles and HPS were employed as chemosensors for the detection of nitro explosives, and o-TS showed the highest sensing ability.


 Please wait while we load your content...
Please wait while we load your content...