Anion binding by p-aminoazobenzene-derived aromatic amides: spectroscopic and electrochemical studies†
Abstract
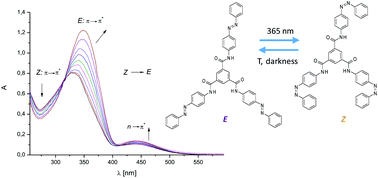
The synthesis and complexing properties of p-aminoazobenzene-derived mono-, bis-, and trisamides were described. Ligands 3 and 4 bind anions, including fluorides, chlorides, bromides, acetates, benzoates, dihydrogen phosphates, hydrogen sulfates, and p-toluenesulfonates, in chloroform forming 1 : 1 complexes. The highest value of stability constant was evaluated for the 4-F− complex (log K = 5.63 ± 0.21). On the basis of 1H NMR, and FTIR spectroscopy, the possible nature of the ligand–anion interactions was proposed. The E ⇄ Z isomerization process of tripodal amide 4 in chloroform was studied. The effect of anions on Z to E thermal back isomerization was investigated.


 Please wait while we load your content...
Please wait while we load your content...