Stereospecific photochemistry of Δ2-1,2,3-triazolines in solution and in the solid state: scope and mechanistic studies†
Abstract
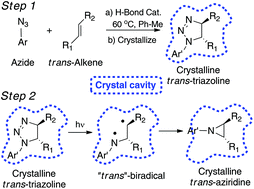
The stereospecific photochemistry of ten N-aryl-substituted cis- or trans-Δ2-1,2,3-triazolines to form the corresponding cis- or trans-aziridines was investigated both in solution and in the solid-state. We found that photochemical reactions in the solid state are more stereospecific than in solution for the 8 crystalline Δ2-1,2,3-triazolines. Additionally, triplet sensitization for some triazolines results in triplet biradicals, which provide the more thermodynamically favored trans-aziridine regardless of the starting triazoline stereochemistry. Product analyses as a function of temperature and solvent polarity suggest that the electronic excitation of the Δ2-1,2,3-triazolines results in the formation of a 1,3-biradical intermediate.


 Please wait while we load your content...
Please wait while we load your content...