Dynamics of excited-state intramolecular proton-transfer in 2-amino-3-(2′-benzazolyl)quinoline cations
Abstract
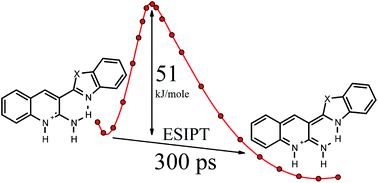
It was found that cations formed by the protonation of 2-amino-3-(2′-benzoxazolyl)-quinoline (ABO) and 2-amino-3-(2′-benzothiazolyl)-quinoline (ABT) at the nitrogen atom of the quinoline ring exhibit excited-state intramolecular proton transfer (ESIPT). The two-band fluorescence of these cations is due to the emission from two species: the initial tautomer (short-wavelength band) and the ESIPT product (long-wavelength band). The relative intensity of the long-wavelength band depends on the basicity of the proton-accepting moiety and temperature. Quantum-chemical calculations demonstrated that ESIPT in cations involves overcoming a significant potential barrier, which increases with the decreasing basicity of the proton-accepting benzazole moiety. Using femtosecond absorption spectroscopy and nanosecond fluorescence spectroscopy, the effective ESIPT time in the studied cations was determined, which increased with decreasing temperature.


 Please wait while we load your content...
Please wait while we load your content...