A light harvesting perylene derivative – zinc phthalocyanine complex in water: spectroscopic and thermodynamic studies†
Abstract
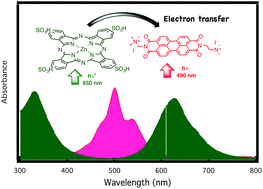
A perylene derivative, namely N,N′-bis(2(trimethylammonium iodide)ethylene)perylene-3,4,9,10-tetracarboxyldiimide (TAIPDI) forms nanoscale columnar stacks in water that have been characterized by using optical absorption and emission measurements, dynamic light scattering (DLS), and transmission electron microscopy (TEM). This behaviour was compared with that of unstacked TAIPDI in methanol. Assembly formation between the one-dimensional TAIPDI stacks and zinc phthalocyanine tetrasulphonic groups (ZnPcS4) via strong π–π and ionic interactions has been described in an aqueous medium. The formation constant of the supramolecular dyad has been determined as 2.94 × 104 M−1 from both the absorption and fluorescence measurements. Upon addition of ZnPcS4, the fluorescence quenching of the singlet-excited state of TAIPDI was observed because of the electron transfer process from ZnPcS4 to TAIPDI via the singlet-excited states of ZnPcS4 and TAIPDI entities. The electrochemical studies supported the electron transfer pathways via the singlet states of ZnPcS4 and TAIPDI. The thermodynamic parameters of the supramolecular complex have been determined from stopped-flow measurements. The interaction between ZnPcS4 and TAIPDI occurs in two steps, where the rate constant of the second step with TAIPDI (207 ± 8 M−1 s−1) is much slower than the first one (3515 ± 101 M−1 s−1). Activation parameters for the complex formation (ΔH# = 76 ± 11 kJ mol−1 and ΔS# = 83 ± 37 J K−1 mol−1, and ΔH# = 221 ± 15 kJ mol−1 and ΔS# = 540 ± 50 J K−1 mol−1) were determined from variable temperature studies for the first and second steps, respectively. The significantly positive ΔS# values found for both steps of the interaction reactions are consistent with a dissociative mechanism.


 Please wait while we load your content...
Please wait while we load your content...