The effect of natural iron oxide and oxalic acid on the photocatalytic degradation of isoproturon: a kinetics and analytical study
Abstract
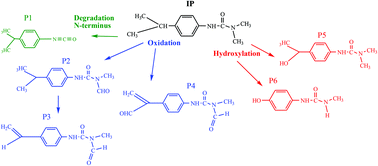
The photocatalytic degradation of isoproturon, a persistent toxic herbicide, was investigated in the presence of natural iron oxide and oxalic acid and under UV irradiation. The influence of the relevant parameters such as the pH and the iron oxide and oxalic acid concentrations has been studied. The presence of natural iron oxide and oxalic acid in the system effectively allow the degradation of isoproturon, whereas the presence of t-butyl alcohol adversely affects the phototransformation of the target pollutant, thus indicating that an OH radical initiated the degradation mechanism. The degradation mechanism of isoproturon was investigated by means of GC-MS analysis. Oxidation of both the terminal N–(CH3)2 and isopropyl groups is the initial process leading to N-monodemethylated (NHCH3), N-formyl (N(CH3)CHO), and CHCH3OH as the main intermediates. The substitution of the isopropyl group by an OH group is also observed as a side process.


 Please wait while we load your content...
Please wait while we load your content...