Photochemical synthesis and photophysical properties of coumarins bearing extended polyaromatic rings studied by emission and transient absorption measurements†
Abstract
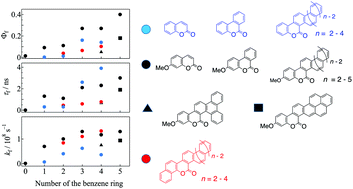
We prepared a variety of coumarin derivatives having expanded π-electron systems along the direction crossing the C3–C4 bond of the coumarin skeleton via a photochemical cyclization process and investigated their photophysical features as a function of the number (n) of the added benzene rings based on emission and transient absorption measurements. Upon increasing n, the fluorescence quantum yields of the π-extended coumarins increased. Expanding the π-electron system on the C3–C4 bond of the coumarin skeleton was found to be efficient for increasing the fluorescence ability more than that on the C7–C8 bond. Introducing the methoxy group at the 7-position was also efficient for enhancing the fluorescence quantum yield and rate of the expanded coumarins. The non-radiative process from the fluorescence state was not substantially influenced by the expanded π-electron system. The competitive process with the fluorescence was found to be intersystem crossing to the triplet state based on the observations of the triplet–triplet absorption. The effects of the expanded π-electron systems on the fluorescence ability were investigated with the aid of TD-DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...