Synthesis and photophysical properties of extended π conjugated naphthalimides†
Abstract
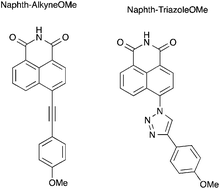
A series of π conjugated naphthalimide derivatives having an imide group as an acceptor conjugated with a methoxy arylethynyl or a methoxyphenyl triazole as a donor were prepared by Sonogashira coupling or “click” chemistry. Their photophysical properties were investigated by steady state and time resolved fluorescence spectroscopy and modelled by TD-DFT calculations. Compound Naphth-AlkyneOMe has a high fluorescence quantum yield and displays efficient photoinduced charge transfer in solution as well as in the powder state. Compound Naphth-TriazoleOMe exhibits a very high Stokes shift and its fluorescence quantum yield is low, which can be rationalized by theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...