Mathematical description of pH-stat kinetic traces measured during photochemical quinone decomposition†
Abstract
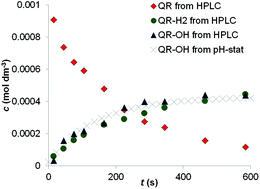
Substituted 1,4-benzoquinone (QR) derivatives are photosensitive in aqueous solution and form hydroquinones (QR-H2) and hydroxy-quinones (QR-OH), two weak acids in their photoreaction. For this reason, the kinetics of the photoreaction can be conveniently followed by the pH-stat titration technique. The mathematical description of the kinetic traces measured provides the two main parameters of the photoreaction: the differential quantum yield of the reaction (Φ) and the ratio of the two photo-products, i.e. the fraction of QR that is converted to QR-OH (α). These values are described in this paper for 2,5-dichloro-1,4-benzoquinone at different pH values, together with the detailed mathematical evaluation of the application limits of the pH-stat method for such reactions.

 Please wait while we load your content...
Please wait while we load your content...