CO2 reduction over NaNbO3 and NaTaO3 perovskite photocatalysts
Abstract
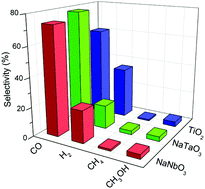
The activity of NaNbO3 and NaTaO3 perovskites for the photocatalytic reduction of CO2 is studied in this work. For this purpose, sodium niobate and tantalate have been prepared using solid-state reactions, extensively characterised by means of powder X-ray diffraction, UV-vis, photoluminescence and Raman spectroscopies and N2 adsorption isotherms, and tested in the gas-phase reduction of CO2 under UV light in a continuous flow photoreactor. NaNbO3 is constituted of an orthorhombically distorted perovskite structure, while a ca. 4.5 : 1 combination of the orthorhombic and monoclinic modifications is found in the tantalate. Both catalysts exhibit interesting intrinsic activities, with the tantalate material giving rise to a slightly higher performance. This is attributed to a compromise situation between electron–hole recombination and the reducing potential of conduction band electrons. In addition, a decrease in the competition of water protons for photogenerated electrons is observed with both catalysts with respect to TiO2.
- This article is part of the themed collection: Solar chemistry & photocatalysis – environmental applications

 Please wait while we load your content...
Please wait while we load your content...